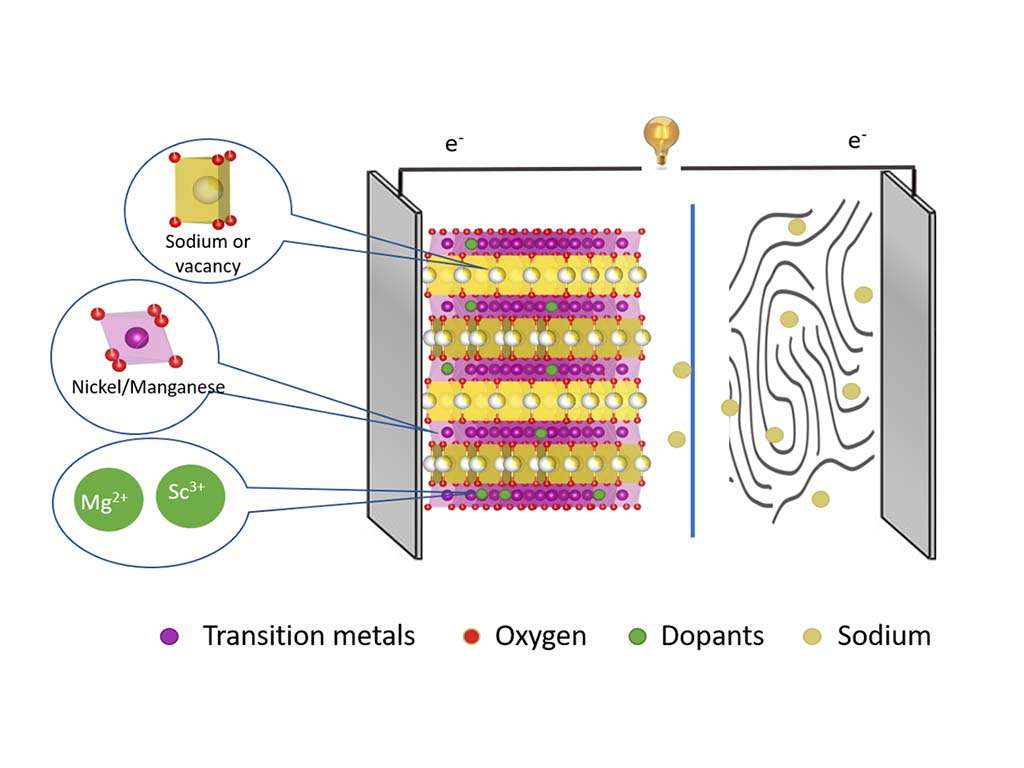
Sodium-ion batteries (SIBs) are a viable alternative to lithium-ion batteries due to the abundant supply of sodium and improving energy density. The abundance of elements such as Na, Fe, and Mn makes SIBs a particularly attractive alternative for grid and large-scale energy storage applications. However, to meet the growing demand for energy storage, higher energy density is essential.
Related articles:
- 2024: A Big Year for Sodium-Ion Batteries?
- The Future Battery Technology Beyond Traditional Lithium-Ion
- Is Sodium Powering the Future?
Optimizing cathode materials through doping with foreign elements like Scandium (Sc) and Magnesium (Mg) can potentially address SIBs’ weaknesses, such as capacity and stability issues. A team from Helmholtz Centre Berlin for Material and Energy (HZB) and Humboldt University Berlin conducted extensive research to understand how doping affects the stability of cathodes made from layered transition metal oxides, specifically using nickel- and manganese-oxide (NMO cathodes).
Cation doping, or more specifically ion substitution, is an effective and practical strategy for achieving superior properties in cathode materials. It has been widely demonstrated in both laboratory experiments and commercial products. The research involved three years of experiments across different X-ray sources (BESSY II, PETRA III, SOLARIS) to analyze the effects of Sc and Mg doping, utilizing techniques like resonant inelastic X-ray scattering (RIXS), X-ray absorption spectroscopy (XAS), X-ray diffraction (XRD), and pair distribution function analysis.
Cation doping with Mg and Sc was found to significantly enhance the cycling stability of Na0.67Ni0.33Mn0.67O2 layered oxides for sodium-ion batteries, by inducing different behaviors in the high voltage region and affecting oxygen redox activities, with Mg showing a particularly strong suppression of oxygen redox activity, challenging previous understandings of its role in Mn-based layered oxides.
Surprisingly, scandium doping did not improve stability as expected. It reduced structural changes during the electrochemical cycle but did not enhance the cathode material’s cycling performance significantly.
Mg doping, on the other hand, is found to reduce oxygen redox activity significantly, a surprising result given Mg’s known role in promoting oxygen redox in other contexts. This suggests a competing mechanism between Ni and Mg affecting oxygen redox behavior.
The electrochemical behavior and oxygen redox activity in Ni–Mn based layered oxides are heavily influenced by the concentration of Mg doping, revealing a balance between enhancing performance and suppressing unwanted high voltage plateaus.
The results suggest that careful selection and concentration of dopants can significantly enhance the performance and stability of layered oxide cathodes for sodium-ion batteries, which is a promising strategy for the improvement of energy storage technologies. This research opens new ways to design more efficient and stable sodium-ion batteries by focusing on the detailed material design of cathodes, especially through the strategic doping of elements such as magnesium.
Source: