Scientists at the U.S. Department of Energy’s Argonne National Laboratory have made significant strides in advancing sodium-ion batteries, offering a potential alternative to lithium-ion batteries for electric vehicles and grid energy storage. By preventing the formation of cracks in cathode particles during the synthesis process, they have addressed a major obstacle to the commercialization of sodium-ion batteries.
Lithium-ion batteries have long been the standard for electric vehicles and are increasingly used for renewable energy storage on the electric grid. However, with the rapid expansion of this market, lithium supply shortages are projected within the next five to ten years. “Sodium-ion batteries are emerging as a compelling alternative due to the greater abundance and lower cost of sodium,” said Gui-Liang Xu, a chemist at Argonne.
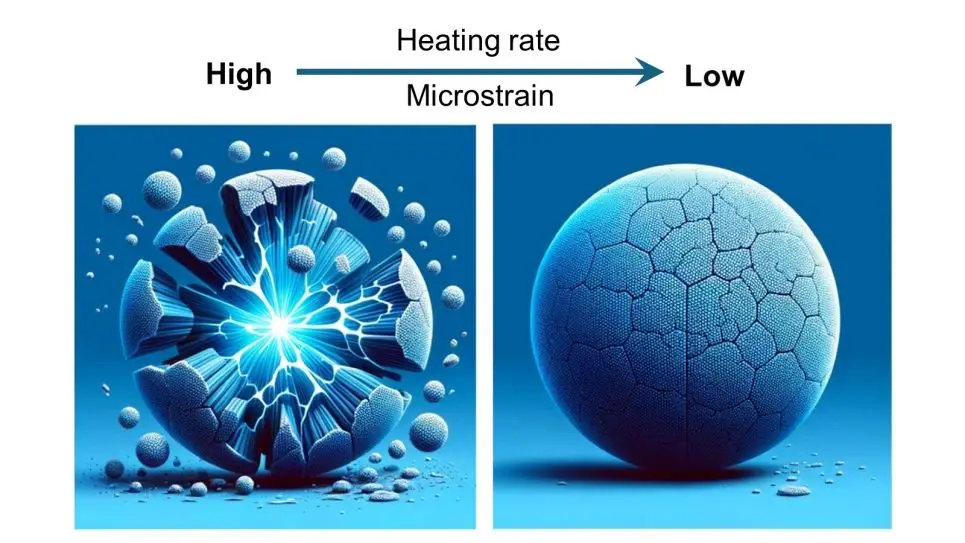
A significant challenge has been the rapid decline in performance of sodium-containing cathodes during repeated charge and discharge cycles. This decline was traced to the formation of cracks in the cathode particles, caused by strain between the core and shell of the particles during cycling.
The Argonne team developed a new design for a sodium-ion oxide cathode, inspired by an earlier successful design for a lithium-ion oxide cathode. The key feature of both designs is that the microscopic cathode particles contain a mix of transition metals—such as nickel, cobalt, iron, or manganese—that are not uniformly distributed. A nickel-rich core provides high energy storage capacity, while a manganese-rich shell offers structural stability.
To eliminate the strain causing cracks, the researchers fine-tuned their cathode preparation method. They discovered that controlling the heat-up rate during synthesis was critical. By slowing the heat-up rate from five degrees Celsius per minute to one degree per minute, they prevented crack formation in the gradient particles. Tests showed that cathodes prepared with the slower heat-up rate maintained high performance over 400 cycles.
“Preventing cracks during cathode synthesis pays big dividends when the cathode is later charged and discharged,” Xu noted. While sodium-ion batteries currently lack the energy density for long-distance electric vehicle travel, they are ideal for urban driving and grid storage applications.
The team is now working to eliminate nickel from the cathode to further reduce costs and enhance sustainability. “The prospects seem very good for future sodium-ion batteries with not only low cost and long life but also energy density comparable to that of the lithium iron phosphate cathode now in many lithium-ion batteries,” said Khalil Amine, an Argonne Distinguished Fellow. “This would result in more sustainable electric vehicles with good driving range.”