Cornell researchers have created a porous crystal that can absorb lithium-ion electrolytes and facilitate smooth ion transport through one-dimensional nanochannels, which could enhance the safety of solid-state lithium-ion batteries.
The findings are detailed in the paper “Supramolecular Assembly of Fused Macrocycle-Cage Molecules for Fast Lithium-Ion Transport,” recently published in the Journal of the American Chemical Society. The research was led by Yu Zhong, assistant professor of materials science and engineering, with Yuzhe Wang ’24, who is the paper’s lead author.
Zhong, whose lab focuses on synthesizing “soft” and nanoscale materials to advance energy storage and sustainability, began this project two years after joining Cornell’s faculty. Wang, an undergraduate transfer student eager to take on a research project, joined Zhong’s team and contributed to the design and development of the crystal structure.
Designing Safer Lithium-Ion Batteries
A primary focus of Yu Zhong’s research has been improving lithium-ion battery safety. In conventional lithium-ion batteries, ions are transported through liquid electrolytes, which can sometimes lead to the formation of dendrites—spiky structures between the anode and cathode that may cause short circuits or, in rare instances, explode.
While solid-state batteries offer enhanced safety, they pose challenges as ions move more slowly through solid materials due to increased resistance. To address this, Zhong set out to develop a porous crystal structure that would enable smoother ion transport along a controlled pathway. This design minimizes interactions between lithium ions and the crystal structure, helping to prevent ion adhesion and ensuring a high concentration of ions for efficient conductivity.
Innovative Molecular Fusion
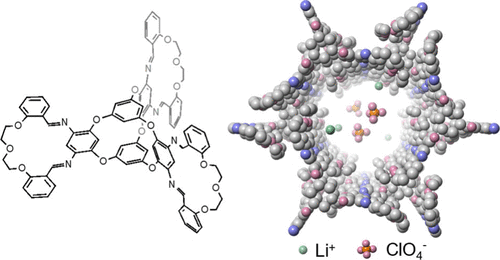
With support from Cornell’s Engineering Learning Initiatives, Yuzhe Wang developed a method to fuse two unique molecular structures—macrocycles and molecular cages—with complementary shapes. Macrocycles are molecules with rings of 12 or more atoms, while molecular cages are multi-ringed compounds with structural pores.
“Both macrocycles and molecular cages have intrinsic pores where ions can sit and pass through,” Wang explained. “By using them as the building blocks for porous crystals, the crystal would have large spaces to store ions and interconnected channels for ions to transport.”
Achieving Record High Ionic Conductivity
Wang designed a structure with a molecular cage at the center and three radially attached macrocycles, forming macrocycle-cage molecules. These molecules use hydrogen bonds and interlocking shapes to self-assemble into complex, nanoporous, three-dimensional crystals with one-dimensional channels – “the ideal pathway for the ion to transport,” according to Zhong. This design achieves ionic conductivity of up to 8.3 × 10⁻⁴ siemens per centimeter.
“That conductivity is the record high for these molecule-based, solid-state lithium-ion-conducting electrolytes,” Zhong noted.
Detailed Structural Analysis and Applications
To better understand the crystal’s structure, the research team collaborated with Judy Cha, Ph.D. ’09, a professor of materials science and engineering used scanning transmission electron microscopy, and Jingjie Yeo, an assistant professor of mechanical and aerospace engineering, provided simulations to clarify interactions between the molecules and lithium ions.
“So with all the pieces together, we eventually established a good understanding of why this structure is really good for ion transport and why we get such a high conductivity with this material,” Zhong explained.
Beyond enhancing lithium-ion battery safety, this material could also be used for ion and molecule separation in water purification, as well as in mixed ion-electron-conducting structures for bioelectronic circuits and sensors.
Exploring Future Applications
“This macrocycle-cage molecule is definitely something new in this community,” Zhong noted. “The molecular cage and macrocycle have been known for a while, but how you can really leverage the unique geometry of these two molecules to guide the self-assembly of new, more complicated structures is kind of an unexplored area. Now in our group, we are working on the synthesis of different molecules, how we can assemble them and make a molecule with a different geometry, so we can expand all the possibilities to make new nanoporous materials. Maybe it’s for lithium-ion conductivity or maybe for even many other different applications.”
Publication: Wang, Y., Wang, K., Ai, Q., Funni, S. D., Garudapalli, A., Fang, Q., Choi, S., Yan, G., Louie, S., Liu, C., Lou, J., Cha, J. J., Yeo, J., Jin, Z., & Zhong, Y. (2024). Supramolecular Assembly of Fused Macrocycle-Cage Molecules for Fast Lithium-Ion Transport. Journal of the American Chemical Society, 146(37), 25433-25438. https://doi.org/10.1021/jacs.4c08558
Source: SciTechDaily