Researchers at the Paul Scherrer Institute (PSI) have developed a novel, sustainable process that enhances the electrochemical performance of lithium-ion batteries. By stabilizing the surface of the battery’s cathode with a thin, uniform protective layer, they have enabled batteries to operate at higher voltages, thereby increasing their energy density without compromising longevity.
Lithium-ion batteries are essential to the decarbonization of various sectors, including transportation and energy storage. One avenue to improve these batteries is to increase their operating voltage, which directly optimizes energy density. However, operating at voltages above 4.3 volts has traditionally led to significant degradation at the interface between the cathode and the electrolyte. This degradation, caused by chemical and electrochemical processes, damages the cathode surface, increases cell resistance, and decreases capacity over time.
The PSI team, led by Dr. Mario El Kazzi from the Center for Energy and Environmental Sciences, addressed this challenge by developing a new coating method for the cathode material. They utilized trifluoromethane (CHF₃), a by-product gas from the manufacturing of plastics like PTFE and PVDF, which is also a potent greenhouse gas. By reacting CHF₃ with the thin layer of lithium carbonate on the cathode surface at 300 degrees Celsius, they converted it into lithium fluoride (LiF), forming a stable protective layer.
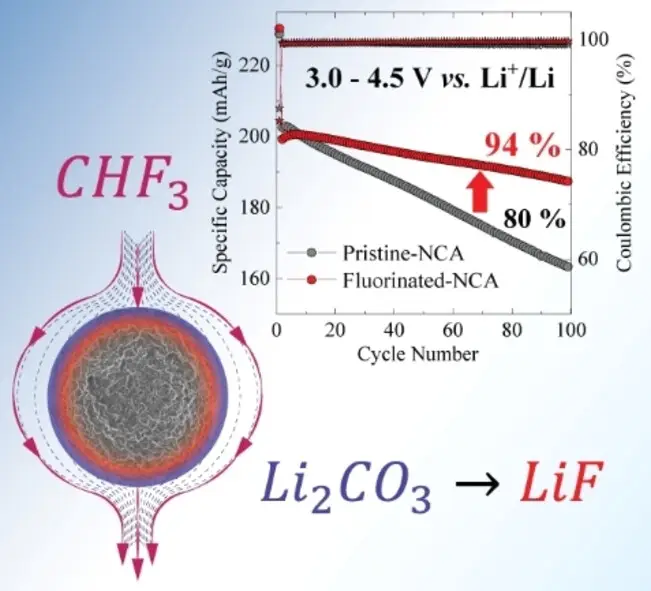
This LiF coating effectively prevents degradation processes at the cathode-electrolyte interface, allowing the batteries to operate at higher voltages of up to 4.8 volts. In electrochemical tests, batteries with the coated cathodes exhibited significantly improved performance compared to uncoated counterparts. After 100 charge-discharge cycles, the coated batteries showed a capacity retention of over 94%, compared to only 80% for uncoated batteries. Additionally, the impedance, or resistance to lithium-ion flow at the cathode interface, was about 30% lower in the coated batteries.
An added environmental benefit of this process is the utilization of CHF₃, which has a global warming potential more than 10,000 times that of carbon dioxide. By converting CHF₃ into a stable component of the battery, the process not only improves battery performance but also provides a means to recycle and sequester this harmful greenhouse gas.
Dr. El Kazzi emphasized the versatility of the LiF protective coating, noting that it could be applied to various cathode materials, including nickel- and lithium-rich high-voltage batteries. “We can assume that our lithium fluoride protective coating is universal and can be used with most cathode materials,” he stated.
This advancement holds promise for increasing the efficiency of lithium-ion batteries, particularly in electric vehicles, leading to longer range and better performance. The findings were published in the journal ChemSusChem, highlighting a significant step toward more sustainable and efficient energy storage solutions.
Converting the CHF3 Greenhouse Gas into Nanometer-Thick LiF Coating for High-Voltage Cathode Li-ion Batteries Materials, Aleš Štefančič, Carlos Antonio Fernandes Vaz, Dominika Baster, Elisabeth Müller, Mario El Kazzi; ChemSusChem (Wiley), 03.01.2025; DOI: 10.1002/cssc.202402057
Source: Paul Scherrer Institute PSI
















